Europe In-Vitro Diagnostics Market Poised for Strong Growth Driven by Rising Cancer and Chronic Disease Cases (2025–2034)
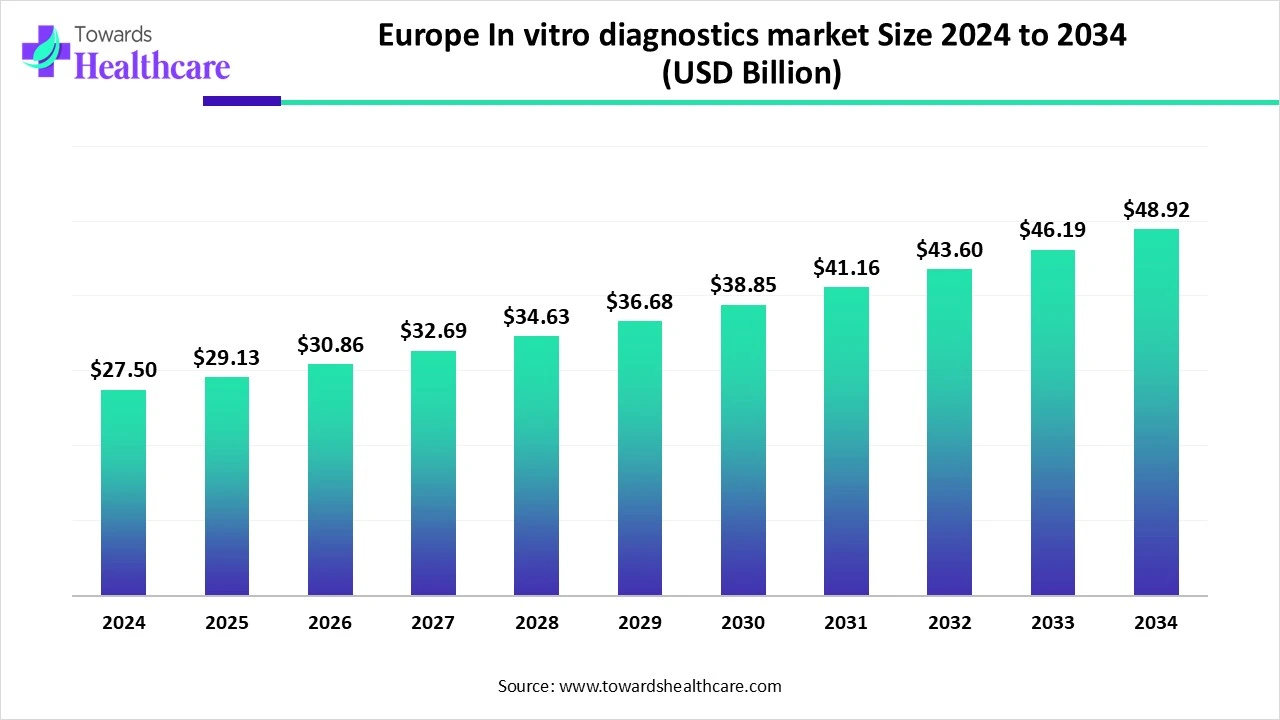
The Europe in-vitro diagnostics market size was valued at USD 29.13 billion in 2025 and is predicted to hit around USD 48.92 billion by 2034, rising at a 5.93% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
Ottawa, Feb. 09, 2026 (GLOBE NEWSWIRE) -- The Europe in-vitro diagnostics market size is calculated at USD 30.86 billion in 2026 and is expected to reach around USD 48.92 billion by 2034, growing at a CAGR of 5.93% for the forecasted period.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5622
Key Takeaways
- Europe in-vitro diagnostics sector is pushing the market to USD 27.5 Bn in 2024.
- Long-term projections show USD 48.92 Bn valuation by 2034.
- Growth is expected at a steady CAGR of 5.93% in 2025.
- The global in vitro diagnostics market is expected to reach $123.45 billion by 2034, growing at a CAGR of 4.45%.
- By product, the reagents segment was dominant in the market in 2024.
- By product, the services segment is expected to grow at the fastest CAGR in the upcoming years.
- By technology, the immunoassay segment registered dominance in the market in 2024.
- By technology, the microbiology segment is expected to witness rapid expansion during 2025-2034.
- By application, the infectious diseases segment dominated the market in 2024.
- By application, the oncology segment is expected to be the fastest-growing during the forecast period.
- By end-use, the hospitals segment led the market in 2024.
- By end-use, the homecare segment is expected to register the fastest growth in the studied years.
What are the Key Factors Impacting Europe In Vitro Diagnostics?
The Europe in-vitro diagnostics market has been progressing due to the emergence of diverse, faster, accurate and automated diagnostic tools, like AI-enhanced algorithms & molecular diagnostics (PCR), with higher effectiveness and minimal operational spending. Also, Europe healthcare system is stepping towards decentralized testing and early diagnosis, empowered by government incentives. For example, recently, Aiforia Technologies received IVDR certification for AI models to automate breast and prostate cancer pathology reads.
What are the Prominent Drivers in the Europe In Vitro Diagnostics Market?
A specific catalyst is the significant rise in incidences of cancers, diabetes, and cardiovascular diseases, which need regular, long-term monitoring and infectious diseases, like respiratory infections, are demanding rapid diagnostic solutions. Alongside, the government is increasingly providing funding to explore innovations in molecular diagnostics, NGS (Next-Generation Sequencing), and liquid biopsy, especially in oncology and infectious disease panels.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What are the Major Trends in the Europe In Vitro Diagnostics Market?
- In December 2025, BD (Becton, Dickinson and Company) expanded its respiratory and sexually transmitted infection (STI) diagnostics offerings in Europe through In Vitro Diagnostic Medical Device Regulation (IVDR) certification of two VIASURE assays introduced by Certest Biotec for use on the BD MAX System.
- In June 2025, FUJIFILM Healthcare Europe & Ibex Medical Analytics partnered to assist in effective and accurate cancer diagnosis.
What is the Crucial Challenge in the Europe In Vitro Diagnostics Market?
Many European companies are facing product withdrawal due to the need for increased expenditure and resource constraints, mainly for small-to-mid-sized manufacturers. Somehow, drained healthcare budgets across Europe are raising pressure on diagnostic reimbursements, which limits the expansion of instrument sales.
Segmental Insights
By product analysis
Which Product Led the Europe In Vitro Diagnostics Market in 2024?
The reagents segment dominated with a major share of the market in 2024. Leaders are increasingly demanding regents in clinical chemistry, molecular diagnostics, and immunoassays. These reagents allow automated, high-throughput testing in labs and rapid POCT, which facilitates vital data in minutes or hours instead of days. Recently, Werfen Aptiva Reagents achieved clearance for increased autoimmune diagnostics optimized specificity in detecting autoimmune diseases.
Furthermore, the services segment is predicted to expand rapidly. This mainly comprises clinical chemistry, molecular diagnostic validation, and automated laboratory analyzers. Current services are stepping towards testing in pharmacies and physician offices, to lower hospital burden. Also, some are boosting the availability and accuracy of self-testing kits for chronic diseases & infectious conditions.
By technology analysis
How did the Immunoassay Segment Dominate the Market in 2024?
In 2024, the immunoassay segment held the largest share of the Europe in-vitro diagnostics market in 2024. Accelerating infectious disease cases are bolstering high-sensitivity Chemiluminescence Immunoassays (CLIA). Whereas, Roche Diagnostics extensively rolled out an innovative AI-driven clinical decision assist tool in Europe, which is united with their existing immunohistochemistry platforms to strengthen cancer diagnostic accuracy.
Moreover, the microbiology segment will expand fastest. Rising instances of respiratory issues, HIV, and hepatitis are highly demanding for rapid, accurate microbiology testing. The latest development include PA-100 AST System (Sysmex Astrego) for transformative, faster, and cost-effective testing for urinary tract infections (UTIs) in primary care, which directly highlights antimicrobial resistance. Whereas, MALDI Biotyper automates sample preparation for near-universal microbial identification by mass spectrometry.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
By application analysis
Which Application Led the Europe In Vitro Diagnostics Market in 2024?
The infectious diseases segment registered dominance in the market in 2024. Ongoing advances in Point-of-care testing are prominently supporting the detection of these issues. Europe is increasingly focused on the progression of multiplex molecular syndromic panels to find 15–20+ respiratory, gastrointestinal, or antimicrobial resistance (AMR) targets in one swab or sample within an hour.
However, the oncology segment is estimated to witness rapid growth. This is primarily propelled by the growing demand for early detection, breakthroughs in liquid biopsy, molecular diagnostics, and the advancing tailored medicine. Recently, the NHS (UK) broadened its Genomic Test Directory to cover ESR1 ctDNA testing for metastatic breast cancer, enabling targeted treatment shifts. Also, Sophia Genetics were located AI platforms to study complex molecular data and estimate tumor response before treatment initiation.
By end-use analysis
Why did the Hospitals Segment Lead the Europe In Vitro Diagnostics Market in 2024?
In 2024, the hospitals segment captured the dominating share of the market. A rise in the ageing population and chronic issues are supporting the wider adoption of IVD solutions in hospitals. Also, these hospitals are experiencing greater, more extensive standards for clinical evidence, especially for high-risk (Class D) diagnostics, which require specialised personnel & resources.
However, the homecare segment will expand rapidly. Patients across Europe are highly prioritizing non-invasive, rapid, and accurate diagnostic tools for home use. In this era, Teal Health raised funds to unveil the Teal Wand, a novel at-home cervical cancer screening kit. Additionally, companies are leveraging AI integration into home diagnostics to boost accuracy & offer instant results to users. Validic introduced a new GenAI solution integrated with Electronic Health Records (EHR) to promote remote patient monitoring (RPM).
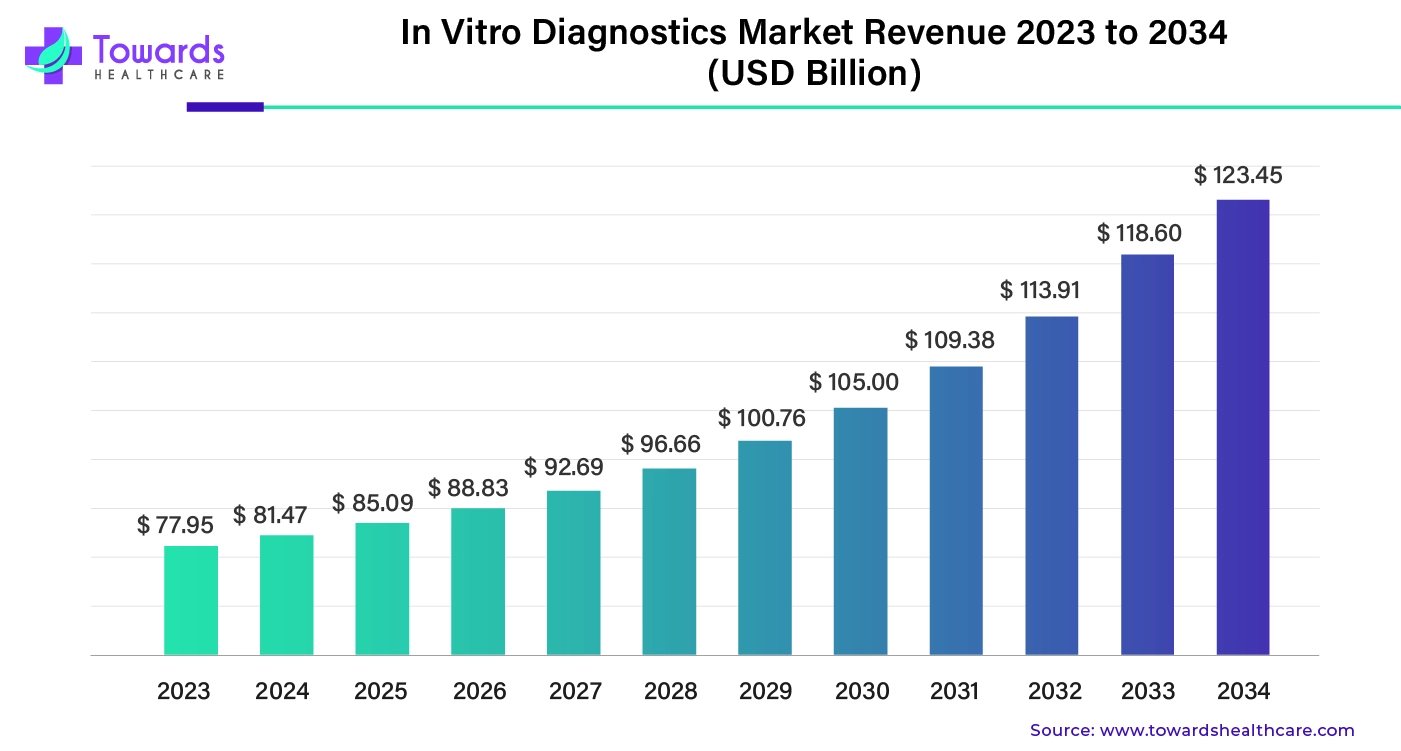
Global In Vitro Diagnostics Market Growth
The global in vitro diagnostics market size was worth about $77.95 billion in 2023. It's expected to grow consistently over the next decade and reach nearly $123.45 billion by 2034, with an average yearly growth rate of 4.45%.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Recent Developments in the Europe In Vitro Diagnostics Market
- In January 2026, Agilent Technologies Inc. unveiled the Agilent S540MD Slide Scanner System, a whole slide imaging digital scanner in substantial European markets.
- In January 2026, the University of Cambridge inaugurated the Institute for Biomedical Innovation (IBI), focused on accelerating the development of medical technologies from laboratory research to clinical testing and real-world use.
Europe In Vitro Diagnostics Market Key Players List

- Bio-Rad Laboratories, Inc
- Abbott
- Sysmex Corporation
- BD
- BIOMÉRIEUX
- Danaher
- F. Hoffmann-La Roche Ltd
- Siemens
- QIAGEN
- Thermo Fisher Scientific Inc.
Browse More Insights of Towards Healthcare:
The global multiplex PCR kit market size is estimated at US$ 1.25 billion in 2024 and is projected to grow to US$ 1.38 billion in 2025, reaching around US$ 3.43 billion by 2034. The market is projected to expand at a CAGR of 10.64% between 2025 and 2034.
The Pre-Dx oncology market is on an upward trajectory, poised to generate substantial revenue growth, potentially climbing into the hundreds of millions over the forecast years from 2025 to 2034.
The global next generation cancer diagnostics market size is estimated at USD 19.20 billion in 2025, grew to USD 20.75 billion in 2026, and is projected to reach around USD 41.64 billion by 2035. The market is expected to expand at a CAGR of 8.05% between 2026 and 2035.
The global ultra-high-end CT scanners market size is calculated at US$ 5.89 billion in 2024, grew to US$ 6.25 billion in 2025, and is projected to reach around US$ 10.69 billion by 2034. The market is expanding at a CAGR of 6.16% between 2025 and 2034.
The global cancer diagnostics market size is calculated at USD 109.65 billion in 2024, grow to USD 116.42 billion in 2025, and is projected to reach around USD 199.54 billion by 2034, rising at a 6.17% CAGR for the forecasted period of 2025 to 2034.
The DNA diagnostics market size was estimated at US$ 10.69 billion in 2023 and is projected to grow to US$ 17.44 billion by 2034, rising at a compound annual growth rate (CAGR) of 4.55% from 2024 to 2034.
The global NGS kits market size is calculated at USD 1.91 billion in 2024, grows to USD 2.26 billion in 2025, and is projected to reach around USD 10.02 billion by 2034. The market is expanding at a CAGR of 18.03% between 2025 and 2034.
The North America in-vitro diagnostics market size is calculated at USD 51.4 billion in 2024, grew to USD 54.08 billion in 2025, and is projected to reach around USD 85.21 billion by 2034. The market is expanding at a CAGR of 5.23% between 2025 and 2034.
The U.S. tuberculosis (TB) diagnostics market size was estimated at USD 650.8 million in 2025 and is predicted to increase from USD 687.51 million in 2026 to approximately USD 1126.5 million by 2035, expanding at a CAGR of 5.64% from 2026 to 2035.
The global AI in cancer diagnostics market size is calculated at USD 1.28 billion in 2026 and is expected to be worth USD 2.86 billion by 2035, expanding at a CAGR of 9.35% from 2026 to 2035.
Segments Covered in the Report
By Product
- Reagents
- Services
- Instruments
By Technology
- Immunoassay
- Instruments
- Reagents
- Services
- Microbiology
- Instruments
- Reagents
- Services
- Hematology
- Instruments
- Reagents
- Services
- Clinical Chemistry
- Instruments
- Reagents
- Services
- Molecular Diagnostics
- Instruments
- Reagents
- Services
- Coagulation
- Instruments
- Reagents
- Services
- Others
- Instruments
- Reagents
- Services
By Application
- Infectious Diseases
- Oncology
- Diabetes
- Cardiology
- Nephrology
- Autoimmune Diseases
- Drug Testing
- Others
By End-use
- Hospitals
- Homecare
- Laboratory
- Others
By Region
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/5622
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Towards Packaging | Towards Food and Beverages | Towards Chemical and Materials | Towards Dental | Towards EV Solutions | Healthcare Webwire
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest
Also Read:
https://www.towardshealthcare.com/insights/ai-in-pathology-market-sizing
https://www.towardshealthcare.com/insights/us-oncology-molecular-diagnostics-market-sizing
https://www.towardshealthcare.com/insights/core-clinical-molecular-diagnostics-market-sizing
https://www.towardshealthcare.com/insights/allergy-diagnostics-market-sizing
https://www.towardshealthcare.com/insights/thyroid-cancer-diagnostics-market-sizing
https://www.towardshealthcare.com/insights/allergy-diagnostics-and-therapeutics-market
https://www.towardshealthcare.com/insights/malaria-diagnostics-market-sizing
https://www.towardshealthcare.com/insights/oncology-molecular-diagnostics-market-sizing
https://www.towardshealthcare.com/insights/computed-tomography-ct-scanners-market-sizing
https://www.towardshealthcare.com/insights/ai-in-diagnostics-market-sizing

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

