Copper single atoms drive cleaner, more efficient hydrogen production
FAYETTEVILLE, GA, UNITED STATES, October 12, 2025 /EINPresswire.com/ -- Harnessing sunlight to convert formic acid into hydrogen offers a promising route to clean energy, but achieving high activity and selectivity remains challenging. Researchers have now developed a cocatalyst that introduces single-atom copper sites into tungsten nitride, creating highly active centers that favor hydrogen generation over unwanted byproducts. When combined with a cadmium sulfide photocatalyst, the new system achieved a hydrogen generation rate of 172.69 μmol·h⁻¹ with selectivity approaching 95%. Detailed experimental and theoretical analyses revealed that copper single atoms enhance charge transfer, open electron pathways, and provide novel active sites for hydrogen evolution. This discovery demonstrates a powerful strategy for designing efficient and selective photocatalytic systems for renewable hydrogen production.
Hydrogen is considered a clean fuel for the future, but conventional production methods remain energy-intensive and carbon-emitting. Photoreforming of formic acid, a common biomass derivative and hydrogen carrier, provides a mild and renewable pathway for hydrogen generation. However, traditional semiconductor catalysts such as CdS suffer from low activity and poor selectivity due to competing side reactions, including carbon monoxide formation, which reduce hydrogen purity. Transition-metal nitrides have recently emerged as cocatalysts with improved performance, yet selectivity limitations persist. Based on these challenges, there is a critical need to develop advanced catalysts with engineered active sites to enhance both efficiency and selectivity in hydrogen photoreforming systems.
A research team from Xi’an Jiaotong University, University of South China, and collaborating institutes reported a development (DOI: 10.1002/cey2.70024) in Carbon Energy on June 4, 2025. The study presents a Cu–W₂N₃ cocatalyst containing atomically dispersed copper sites, which, when integrated with CdS, markedly enhances hydrogen production from the photoreforming of formic acid. The new system achieved higher activity and nearly 95% hydrogen selectivity, outperforming conventional cocatalysts. This work sheds light on how tailoring single-atom sites on cocatalyst surfaces can effectively direct reaction pathways toward clean hydrogen generation.
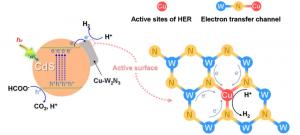
The team synthesized two-dimensional Cu–W₂N₃ nanosheets using a modified molten salt method, ensuring the copper species were atomically dispersed rather than aggregated into nanoparticles. Advanced microscopy and spectroscopy confirmed the presence of Cu single-atom sites with a +1 oxidation state embedded in the W₂N₃ lattice. These sites altered the electronic structure, creating Cu–N–W electron channels that facilitated rapid charge transfer from CdS to the cocatalyst. Performance tests showed that the CdS/Cu–W₂N₃ hybrid achieved a hydrogen generation rate of 172.69 μmol·h⁻¹ with selectivity up to 93.7–95%, significantly higher than bare CdS (45%) or CdS/W₂N₃ (83%). The system also demonstrated excellent stability across ten continuous cycles. Mechanistic studies revealed that copper single atoms suppress carbon monoxide evolution by reducing nitrogen vacancy sites while simultaneously providing efficient new active centers for hydrogen evolution. Density functional theory calculations further supported that Cu single atoms on the {001} facet are energetically favorable for hydrogen formation. Together, these findings confirm that rational design of single-atom cocatalysts is a powerful strategy to simultaneously achieve high activity and selectivity in solar-driven hydrogen production.
“Our study shows that introducing single-atom copper sites into tungsten nitride fundamentally changes the selectivity of photocatalytic reactions,” said Prof. Xiangjiu Guan, corresponding author. “These atomically dispersed sites not only accelerate electron transfer but also suppress competing pathways that produce carbon monoxide. This dual function allows us to obtain hydrogen with very high purity under sunlight. The work highlights how careful engineering of cocatalyst surfaces at the atomic level can unlock new opportunities for efficient, sustainable hydrogen production from renewable resources.”
The development of Cu–W₂N₃ single-atom cocatalysts opens a new pathway toward practical solar-driven hydrogen production. By ensuring high selectivity and stability, the system addresses two major barriers to scaling photocatalytic hydrogen technologies. Beyond formic acid, the design principles could be applied to other biomass-derived fuels and liquid hydrogen carriers, offering a sustainable route for coupling carbon-neutral energy conversion with biomass utilization. The study also advances the broader field of single-atom catalysis, showing how atomic precision can control reaction outcomes. Ultimately, this approach paves the way for producing high-purity hydrogen as a clean fuel for transportation, storage, and chemical industries.
References
DOI
10.1002/cey2.70024
Original Source URL
https://doi.org/10.1002/cey2.70024
Funding Information
This study was supported by the National Natural Science Foundation of China (Grant Nos. 52488201, 52376209, and 22108218), the China Postdoctoral Science Foundation (Grant Nos. 2020M673386, and 2020T130503), and the Fundamental Research Funds for the Central Universities.
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.